4.2Multi-Omics Workflow
Multi-Omics Mechanistic Modeling Approach to Uncovers Novel Mechanisms of Kidney Fibrosis Progression
In this workflow, we demonstrate how multi-omics integration can be leveraged to dissect cellular responses to specific stimuli. Here, we will investigate human kidney PDGFRβ⁺ mesenchymal cells stimulated with TGF-β, a key profibrotic cytokine, to gain mechanistic insights into the development of kidney fibrosis. While the original study employed a time-resolved design to capture the dynamic response of these cells over several time points, this workflow will focus on a single representative time point for simplicity. For readers interested in the full temporal analysis and its biological implications, please check out the original publication (Tuechler, 2025).
Kidney fibrosis is a hallmark of chronic kidney disease (CKD), characterized by the progressive accumulation and restructuring of extracellular matrix (ECM) components, ultimately leading to organ dysfunction and failure (Yamashita, 2024; Kim, 2022). However, despite its clinical relevance and growing prevalence, our molecular understanding of the fibrotic process remains limited.
Here, we leverage transcriptomics, proteomics, phosphoproteomics and secretomics data to uncover mechanistic insights into the fibrotic remodeling of mesenchymal cells which can help to idenfitiy candidate biomarkers and therapeutic targets for kidney fibrosis.
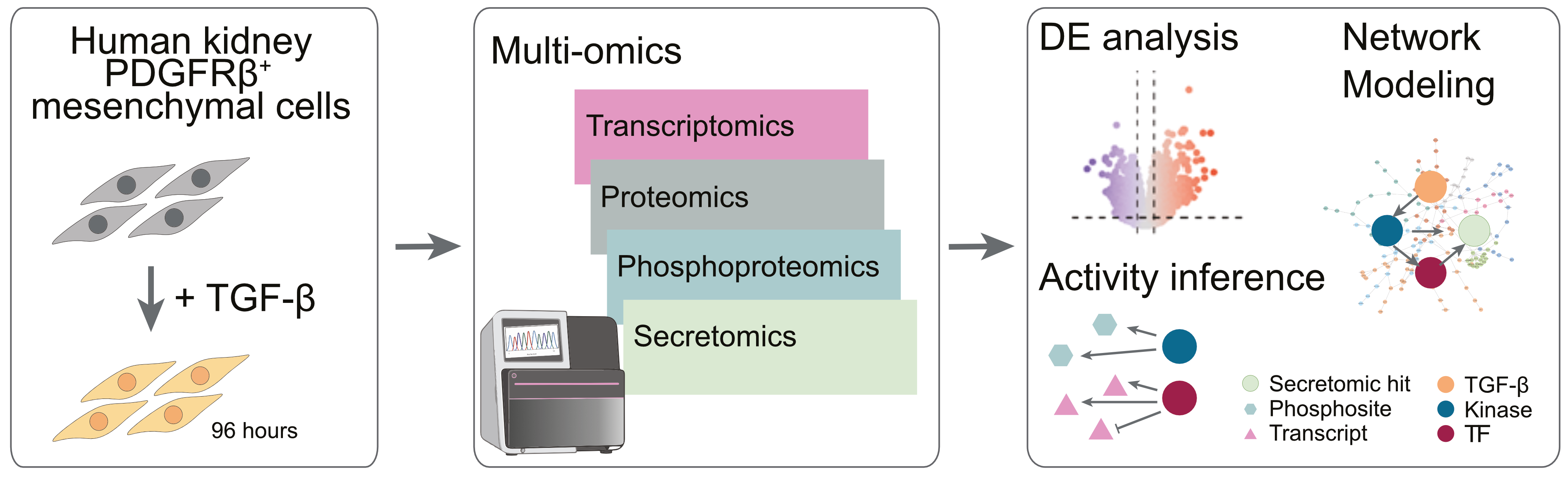
Figure 1:Multi-omics analysis of human kidney PDGFRβ+ mesenchymal cells to investigate fibrosis progression and identifying potentially novel regulatory mechanisms through integrated transcriptomic, proteomic, phosphoproteomic and secretomic profiling.
- Tuechler, N., Burtscher, M. L., Garrido-Rodriguez, M., Khan, M. M., Türei, D., Tischer, C., Kaspar, S., Schwarz, J. J., Stein, F., Rettel, M., Kramann, R., Savitski, M. M., Saez-Rodriguez, J., & Pepperkok, R. (2025). Dynamic multi-omics and mechanistic modeling approach uncovers novel mechanisms of kidney fibrosis progression. Molecular Systems Biology. 10.1038/s44320-025-00116-2
- Yamashita, N., & Kramann, R. (2024). Mechanisms of kidney fibrosis and routes towards therapy. Trends in Endocrinology & Metabolism, 35(1), 31–48. 10.1016/j.tem.2023.09.001
- Kim, K. P., Williams, C. E., & Lemmon, C. A. (2022). Cell–Matrix Interactions in Renal Fibrosis. Kidney and Dialysis, 2(4), 607–624. 10.3390/kidneydial2040055